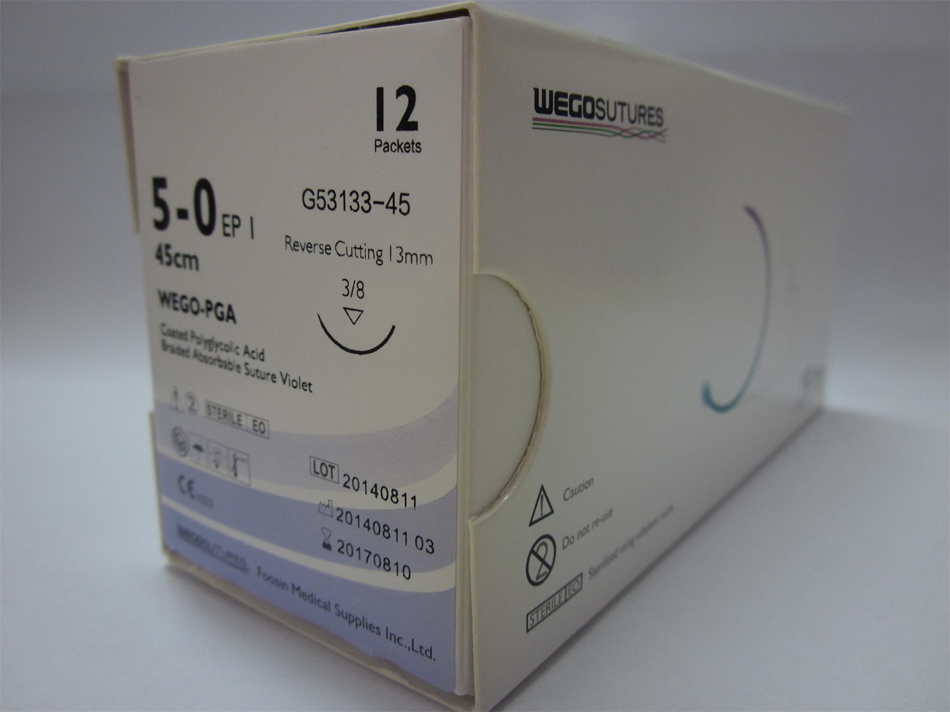
New Packaging PGA Surgical Suture
 Product will finish verification of five years validity
WEGO-PGA Features
Structure Multifilament, braided
Chemical composition 100%polyglycolic acid
Coating Polycaprolactone + Calcium Stearate
Color Violet or Undyed
Sizes USP 2-USP 8/0 metric 5 - metric 0.4
Knot tensile strength Retention 14 days post implantation 60-70%
18 days post implantation 50%
21 days post implantation 40%
Mass absorption Degradation by hydrolysis within 60-90 days
Indications General surgery, Gastroenterology, Urology, Gynaecology, Ophthalmic, Plastic and Paedriatic surgery
Sterilization Ethylene oxide


 CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center.
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot, absorbed by the tissues with hydrolysis after implant, safe and without histological reactions. Sterile by EO conformity with ISO11137 by a CE marked sterilize center. Product will finish verification of five years validity
WEGO-PGA Features
Structure Multifilament, braided
Chemical composition 100%polyglycolic acid
Coating Polycaprolactone + Calcium Stearate
Color Violet or Undyed
Sizes USP 2-USP 8/0 metric 5 - metric 0.4
Knot tensile strength Retention 14 days post implantation 60-70%
18 days post implantation 50%
21 days post implantation 40%
Mass absorption Degradation by hydrolysis within 60-90 days
Indications General surgery, Gastroenterology, Urology, Gynaecology, Ophthalmic, Plastic and Paedriatic surgery
Sterilization Ethylene oxide



The screw system contains cortical screw, cancellous screw and Cannulated Screw. Torx and Hex pattern are available. The material is Alloy Titanium or Stainless Steel. The screw use asymmery thread to increase the holding force. Locking screw use four-thread design for quick screw fixing. The screw has self-taping and self-drilling features.
Various products of Bone Screw, providing product images and basic parameters with each High Quality Bone Screw; We are a professional Chinese manufacturer of Bone Screw, and look forward to your cooperation!
We strive to provide superior benefits to professionals and patients through the development of reliable products.

Bone Screw
Bone Screw,Orthopedic Bone Screws,Cortical Screws,Cannulated Screw
Shandong Hangwei Orthopedics Medcial Instrument Co., Ltd. , http://www.hangweimedical.com